Monkeypox virus(MPV) is from the family Poxviridae,subfamiliy:chordopoxvirinae,genus: orthopoxvirus,and species: Monkeypox virus.
It is a zoonotic disease endemic to central and western Africa and most concentrated in the Democratic Republic of Congo. Although first identified in captive monkeys,the available data suggests infections have occurred in squirrelsrats,mice,monkeysprairie dogs, and humans.
Monkeypox virus is a double -stranded DNA zoonotic virus that causes monkeypox in humans and other animals , and is also a virus belonging to the Poxviridae family . Monkeypox is similar to smallpox , but causes a less severe rash and a lower mortality rate .
The monkeypox virus originating in Central Africa is called the Congo Basin (Central Africa) clade virus, and the other is the West African clade virus.The viruses of the Central African clade were found to be more virulent than those of the West African clade.
Currently,transmission can occur through contact with bodily fluids.skin lesions,or respiratory droplets of infected animals directly or indirectly via contaminated fomites.
Although human-to-human transmission occursdisease spread between humans is limited. Based on the current epidemiological investigation,the incubation period and typically lasts 7 to 14 days with an upper limit of 21 days.Initial symptoms include fever,headache,myalgia,fatigue,and lymphadenopath.After1to 2 days,mucosal lesions develop in the mouth closely by skin lesions of the face and extremities(including palms and soles)and are centrifugally concentrated.
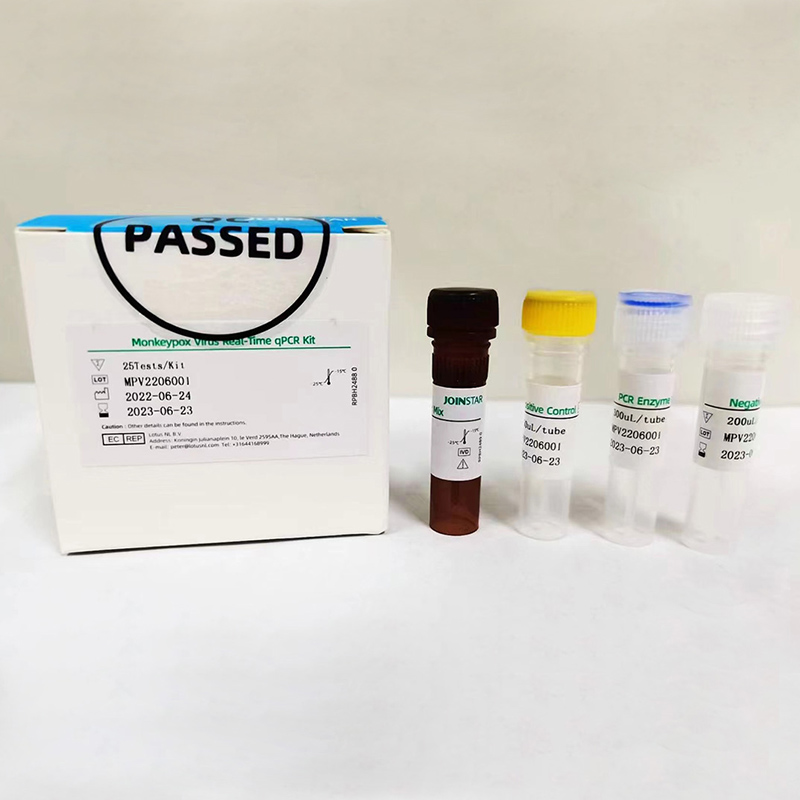
The Monkeypoxvirus Real-Time qPCR Kit is a real- time fluorescence quantitative PCR kit.The Monkeypox virus primer and probe set(s) are designed to simultaneously detect two different targets: one Monkeypox virus F3L genesand one primer and probe set detecting human Housekeeping gene(internal controlic) that is spiked into both the positive control and clinical samples.
The Respiratory or eye secretionsrashscabvesicular fluid, and whole blood specimens should be collected transported and stored according to standard procedures. DNA extracted and purified from patient specimens is amplified in the appropriate real-time fluorescence quantitative PCR Instrument. In the processthe probe anneals to a specific target sequence located between the forward and reverse primers.During the extension phase of the PCR cycle, the 5’nuclease activity of Taq polymerase degrades the probe, causing the reporter dye to separate from the quencher dye, generating a fluorescent signal With each cycle, additional reporter dye molecules are cleaved from their respective probes. Fluorescence intensity is monitored at each PCR cycle.
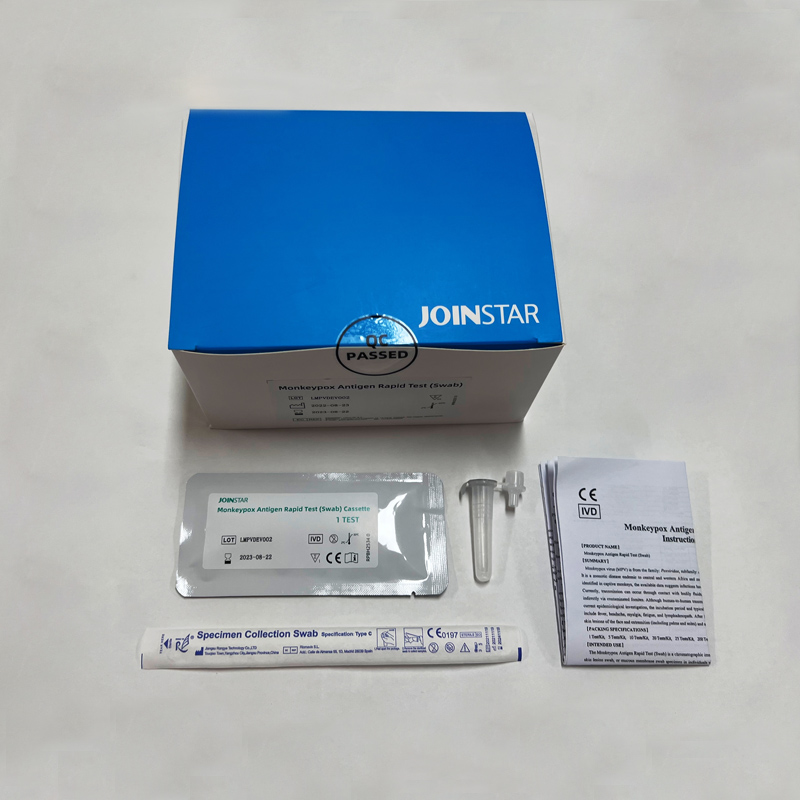
The Monkevpox Antigen Ranid Test(Swab) is a chromatographic immunoassav for the qualitative detection of Monkevpox antigen in humar
skin lesion swab,or mucous membrane swab specimen in individuals who are suspected of monkeypox-like symptoms or recent contact with monkeypox infected patients.【PRINCIPLE】
The Monkeypox Antigen Rapid Test(Swab)is a qualitative membrane strip based on immunoassay for the detection of the Monkeypox
antigen from in human skin lesion swab,or mucous membrane swab specimen. In this test procedure the specimen reacts with the anti
Monkeypox antibody conjugate coated particles on the label pad,and then the mixture migrates upward on the membrane chromatographically by capillary action and reacts with the anti-Monkeypox antibody in the detection region.
If the specimen contains Monkeypox antigen,a colored line will appear in the test line region indicating a positive result.If the specimen does not contain Monkeypox antigen,a colored line will not appear in this region indicating a negative result.To serve as a procedural control, a
colored line will always appear at the control line region indicating that proper volume of specimen has been added and membrane wicking has occurred.